The chemical expression Hcooch ch2 h2o may appear as a set of letters and numbers, but it represents a meaningful concept in organic and general chemistry. The formula points toward the coexistence of a formyl or formate group, a methylene unit, and the ever-essential water molecule. These components are not random; they are fundamental players in many organic reactions. The understanding of hcooch ch2 h2o allows chemists to decode mechanisms, reactions, and practical applications that stretch from classroom examples to industrial-scale processes.
Understanding the Structural Elements of hcooch ch2 h2o
Breaking down the structure of hcooch ch2 h2o reveals three vital units: the HCOO group, the CH2 linkage, and H2O. Each of these units carries unique chemical behavior. The HCOO portion resembles formate, a group linked to carboxylic acids and esters. The CH2 fragment stands for methylene, which is central to hydrocarbons and organic chains. Water, of course, is the universal solvent and one of the most reactive simple molecules. When combined, these groups open doors to multiple reactions and interactions.
Chemical Nature of hcooch ch2 h2o
The chemical nature of hcooch ch2 h2o lies in the interaction between polar and nonpolar forces. The HCOO group is polar, allowing it to engage in ionic or hydrogen bonding. The CH2 part is nonpolar, giving hydrophobic characteristics. Water, being polar, influences the entire behavior of the system by acting as a medium. These combined features create a dynamic balance that chemists exploit in processes such as hydrolysis, esterification, and condensation.
Behavior in Organic Reactions
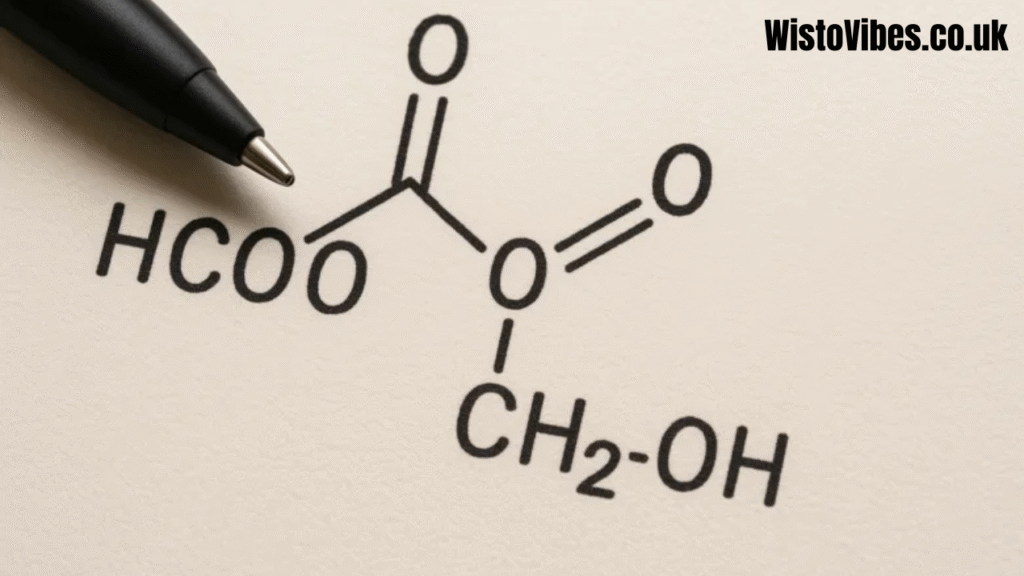
Organic reactions involving hcooch ch2 h2o highlight its versatility. The HCOO group can donate or accept protons, act as a leaving group, or form esters. The CH2 portion can undergo substitution or addition reactions depending on conditions. Water can either break bonds or emerge as a product during condensation. This trio allows reactions to proceed under mild laboratory conditions and also under industrial synthesis routes, making it a key system to study.
Esterification Pathways of hcooch ch2 h2o
Esterification is one of the classic reactions connected to hcooch ch2 h2o. Here, the HCOO group interacts with CH2-related alcohol fragments, often with water either present or released. The reaction produces esters, which are essential in fragrances, solvents, and polymers. Understanding how hcooch ch2 h2o participates in esterification helps both students and professionals see the importance of functional group interactions.
Hydrolysis and hcooch ch2 h2o

Hydrolysis is another major pathway where hcooch ch2 h2o plays a role. In hydrolysis, water becomes the active agent, breaking down esters or other molecules into acids and alcohols. The presence of CH2 units and HCOO groups makes this process especially relevant in organic and biological chemistry. Hydrolysis not only has laboratory importance but also occurs in living organisms, where enzymes catalyze such reactions.
Condensation Reactions with hcooch ch2 h2o
Condensation reactions represent the opposite of hydrolysis. Here, water is released as molecules combine to form larger structures. The system of hcooch ch2 h2o perfectly models this process, as the formyl and methylene units can link, eliminating water as a byproduct. This reaction is essential in building polymers, proteins, and synthetic materials. Thus, hcooch ch2 h2o is central to understanding how small molecules grow into larger, functional systems.
Industrial Importance of hcooch ch2 h2o
Industrially, hcooch ch2 h2o highlights the foundation of chemical manufacturing. Esters derived from such combinations are used in perfumes, food flavorings, and solvents. The methylene group is fundamental in producing plastics and resins. Water is indispensable in moderating reaction conditions and controlling heat. Taken together, the system of hcooch ch2 h2o symbolizes the core of applied organic chemistry that supports modern manufacturing.
Educational Relevance of hcooch ch2 h2o
In education, hcooch ch2 h2o serves as a teaching example for functional group chemistry. It helps students visualize how reactions happen in the presence of water, how esters are formed, and why hydrolysis matters. Teachers often use such combinations to explain the delicate balance between theoretical chemistry and practical experiments. By analyzing hcooch ch2 h2o, learners build a foundation for more complex studies in organic, physical, and analytical chemistry.
Environmental Impact of hcooch ch2 h2o
The environmental perspective on hcooch ch2 h2o focuses on its degradability and safety. Formates and methylene derivatives can appear in waste streams of industries. When water interacts with them, hydrolysis can sometimes reduce toxicity, creating less harmful products. However, without proper treatment, such compounds can contribute to pollution. Understanding the chemistry of hcooch ch2 h2o is therefore vital for designing environmentally friendly disposal and recycling processes.
Biological Significance of hcooch ch2 h2o
Biology makes frequent use of chemical groups similar to hcooch ch2 h2o. The formate group participates in metabolic cycles. Methylene groups form the backbone of DNA, proteins, and lipids. Water is, of course, the solvent of life, enabling all biochemical processes. Together, these elements underscore the biological relevance of hcooch ch2 h2o, reminding us that the principles of organic chemistry directly overlap with living systems.
Research Applications of hcooch ch2 h2o
In research laboratories, hcooch ch2 h2o is a model system for studying kinetics, catalysis, and solvent effects. Scientists use it to understand how catalysts accelerate reactions, how temperature changes affect rates, and how solvents influence stability. Computational chemists also simulate the structure to predict reaction pathways. These applications are essential in designing green chemistry solutions, developing drugs, and improving materials.
Safety Factors in Handling hcooch ch2 h2o
Safety must always be considered when handling chemicals connected to hcooch ch2 h2o. While water is harmless, formates can irritate skin and eyes, and methylene-related compounds may be hazardous. Laboratory protocols such as wearing gloves, goggles, and ensuring ventilation are crucial. Industrially, workers must follow strict safety guidelines to avoid accidents. Understanding the properties of hcooch ch2 h2o is therefore not only a matter of knowledge but also of responsibility.
Industrial Synthesis Routes Involving hcooch ch2 h2o
Industrial synthesis often relies on the principles demonstrated by hcooch ch2 h2o. Large-scale esterification creates solvents and plasticizers. Condensation reactions build polymers used in packaging and textiles. Hydrolysis, when controlled, is employed in food processing and waste management. These routes highlight the economic significance of understanding how hcooch ch2 h2o operates in chemical systems.
Green Chemistry and hcooch ch2 h2o
Green chemistry focuses on designing processes that minimize waste and energy use. hcooch ch2 h2o plays a role here because its components can be manipulated in eco-friendly reactions. Water, as a solvent, is far safer than many organic solvents. Using formates and methylene units in controlled reactions can reduce environmental hazards. Thus, the chemistry of hcooch ch2 h2o contributes to sustainable development goals.
Role in Polymer Science
Polymer science benefits from the reactivity modeled by hcooch ch2 h2o. Condensation and esterification reactions are foundational in creating polymers. Methylene groups provide chain stability, while formyl interactions introduce reactive sites. Water often emerges as a byproduct during polymerization. Understanding this system helps material scientists design stronger, lighter, and more versatile polymers for modern industries.
Comparative Chemistry of hcooch ch2 h2o
Comparing hcooch ch2 h2o with other simple systems such as acetic acid derivatives or ethanol shows both parallels and differences. While formates behave like other carboxylates, their smaller size often makes them more reactive. Methylene is one of the simplest hydrocarbon fragments, yet it has unique properties compared to longer chains. Water remains constant, but its role varies with molecular context. Such comparisons help refine chemical understanding.
Advanced Applications of hcooch ch2 h2o
Advanced applications of hcooch ch2 h2o extend to pharmaceuticals, materials, and biofuels. In pharmaceuticals, esterification pathways inspired by this system are used to design prodrugs. In materials science, polymers based on condensation reactions improve sustainability. In biofuels, methylene and formate chemistry contribute to energy-efficient processes. Thus, hcooch ch2 h2o goes beyond theory to inspire innovation.
Theoretical Importance of hcooch ch2 h2o
From a theoretical perspective, hcooch ch2 h2o represents the balance of polarity, reactivity, and solubility. It models how functional groups interact in the presence of water and how equilibrium shifts between products and reactants. These theoretical lessons apply to nearly every area of chemistry, from physical models to quantum simulations. The study of hcooch ch2 h2o therefore enriches both applied and fundamental sciences.
Conclusion on hcooch ch2 h2o
In conclusion, hcooch ch2 h2o encapsulates the principles of organic chemistry, industrial application, environmental care, and biological relevance. Its parts—formyl groups, methylene units, and water—are simple yet powerful in shaping countless chemical processes. By studying this system, one understands not just specific reactions but the very heart of chemical science. Whether in classrooms, industries, or laboratories, hcooch ch2 h2o remains a symbol of how chemistry connects theory with real-world practice.
Read More: Understanding v4holt: A Comprehensive Exploration




